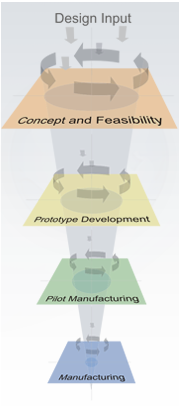
The Stellartech Development Process
Our integrated teams work within a structured Development Process that focuses activities on the achievement of your goals. Each Process Phase has been carefully designed to maximize productivity, and minimize risks, while ensuring compliance with medical regulations.
The four key Stellartech phases are:
- Concept and Feasibility Phase
- Prototype Development Phase
- Pilot Manufacturing Phase
- Manufacturing Phase
Special features of the Stellartech Development Process include:
- Tight Control of Schedules, Costs and Results — We aggressively manage our Development Process to deliver results and carefully control schedules and costs. During your project, we allocate higher levels of manpower during the development phase and reduce manpower during the clinical and regulatory phases to minimize development time and conserve capital, reducing the “burn rate”. If required, we draw on our relationships with expert consultants to give you access to the best talent available.
- Value-Added Activities — We can perform value-added activities at your request, such as helping to guide your regulatory strategy or develop your intellectual property portfolio.
- Continuous Communication — We believe that good communication is fundamental to a sound plan and have built informal interaction as well as formal customer reviews into each Process phase.
- Commitment to Clinical Success — Our broad-based medical device expertise is always at work for you. Throughout all phases of design, development and manufacturing, we constantly challenge ourselves to seek out improvements to optimize your product's performance and enhance clinical results.